Author: Vignesh Subramanian, Class of 2024
Cerebral amyloid angiopathy (CAA) is a cerebrovascular disorder characterized by the accumulation of amyloid protein deposits along cortical capillaries, cerebral vessel walls, and the brain’s leptomeninges. These plaques weaken fragile blood vessels, often resulting in intracerebral hemorrhaging. CAA is linked to vascular cognitive impairment and dysfunction and is a major contributor to the pathogenesis of Alzheimer’s disease (AD) and other neurodegenerative dementias. While the underlying pathophysiology of CAA is not entirely understood, it is believed that cerebrospinal fluid (CSF) flow drains protein plaques and other waste from the cerebrovasculature and that this function deteriorates with age-related inflammation, leading to CAA. This may represent a breakdown in the glymphatic system – the newly discovered waste clearance network of the central nervous system through which CSF flows – that involves the presence of CAA-linked plaques abnormally re-routing CSF currents away from established pathways in the brain.
To better understand how glymphatic system function or ordinary lymphatic drainage may be implicated with CAA, researchers co-led by Dr. Tannenbaum of Stony Brook University analyzed the processes’ CSF flow dynamics. The researchers administered injections of gadoteric acid (Gd-DOTA) to a transgenic adult rat line (called rTg-DI) engineered to robustly develop type I CAA, in which fibrillar amyloid deposits covered 50-75% of the cortical capillaries rather than larger cerebral vessel walls. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was used to follow Gd-DOTA as it tracked the velocity of CSF flow throughout the brain.
The researchers found that in rTg-DI rats with substantial CAA pathology, CSF flows at faster rates through the perivascular spaces of the circle of Willis and middle cerebral arteries, the primary influx routes to the glymphatic system. These high-speed CSF currents were found to be partially redirected away from these routes at the skull base, with resultant decreases of up to 20% in the glymphatic volume flux of CSF passing through and clearing regions with extensive CAA pathology, such as the hippocampus. Furthermore, the researchers found that regular lymphatic function (which is similarly responsible for waste clearance, but for the whole body and primarily while the individual is awake) is also impaired by CAA pathology, with solute drainage via deep cervical lymph nodes being diverted along the carotid arteries in the neck and significantly slowed. The findings suggest that addressing compromised glymphatic transport and lymphatic drainage systems may pave the way for effective treatment of CAA-associated dementia progression.
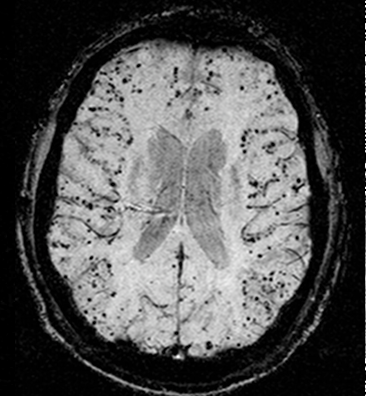
Figure 1: An MRI scan of cerebral amyloid angiopathy (CAA) in a human brain, with associated amyloid-beta peptide deposits appearing as black dots in the brain’s outer layer.
[1] Chen, X., Liu, X., Koundal, S., Elkin, R., Zhu, X., Monte, B., Xu, F., Dai, F., Pedram, M., Lee, H., Kipnis, J., Tannenbaum, A., Van Nostrand, W. E., & Benveniste, H. (2022). Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nature Aging, 2(3), 214-223. doi: 10.1038/s43587-022-00181-4
[2] Image retrieved from: https://simple.m.wikipedia.org/wiki/File:Cerebral_amyloid_angiopathy_%28CAA%29-MRI.png